Cutting Edge Features
- Description
- Specifications
- Shipping Information
- Warranty
Description
About the WHILL Model C2: Award-Winning Power Wheelchair
Often imitated. Never duplicated. The WHILL Model C2 power chair has it all — impressive power, a stylish design, and superior handling thanks to its patented omni-directional wheels. When it comes to portable electric wheelchairs, this device is in a class of its own. With its unique combination of luxury, performance, and innovative technology, the Model C2 gives users more freedom in their everyday lives and sets a new standard for what a mobility device can be.
We designed the WHILL Model C2 to provide users with the ultimate wheelchair experience, allowing you to get more out of life without sacrificing comfort or style. Its award-winning design is a testament to our mission of creating high-quality electric wheelchairs that allow everyone to explore the world with confidence.
FDA Compliance: The WHILL Model C2 has received 510(k) clearance for marketing as a Class II medical device.
Specifications
Performance
| Driving Range with Full Battery Charge | Up to 12.4 miles (20 km) |
| Maximum Speed *1 | 5 mph (8 km/h) 3.7 mph (6 km/h) |
| Ground Clearance | 3 inches (76 mm) |
| Obstacle Clearance | 2 inches (50 mm) |
| Maximum Incline | 10 degrees |
| Turning Radius | 29.9 inches (760 mm) |
| Weight Capacity | 300 pounds (136 kg) |
| Air Travel Approved | Yes |
| Weather Tested | IPX4 |
| Suspension | 4-Wheel Independent |
*1 The maximum speed depends on local regulations.
Size and Weight
| Seat Sizes | W 16 x D 16 inches (W 400 x D 400 mm) W 18 x D 18 inches (W 450 x D 450 mm) W 20 x D 18 inches (W 500 x D 450 mm) |
| Device Width *2 | 21.8 to 25.6 inches (554 to 650 mm) |
| Device Length | 38.8 inches (985 mm) |
| Device Height *3 | 26.4 to 28.7 inches (670 to 730 mm) |
| Device Weight *4 | 114 pounds (51.9 kg) 116 pounds (52.6 kg) 116.4 pounds (52.8 kg) |
| Battery Weight | 6 pounds (2.7 kg) |
| Seat Assembly Weight | 26.9 to 28.9 pounds (12.2 to 13.1 kg) |
| Front Drive Base Weight | 38.8 pounds (17.6 kg) |
| Rear Drive Base Weight | 42.8 pounds (19.4 kg) |
*2 Total device width based on seat width.
*3 At top of controller. Highest point of device with back support folded down.
*4 Includes battery (6 lbs (2.7 kg)). Device weight varies by seat size.
Battery and Power
| Charging Time | Up to 5 hours |
| Battery Type | Lithium-ion |
Adjustments
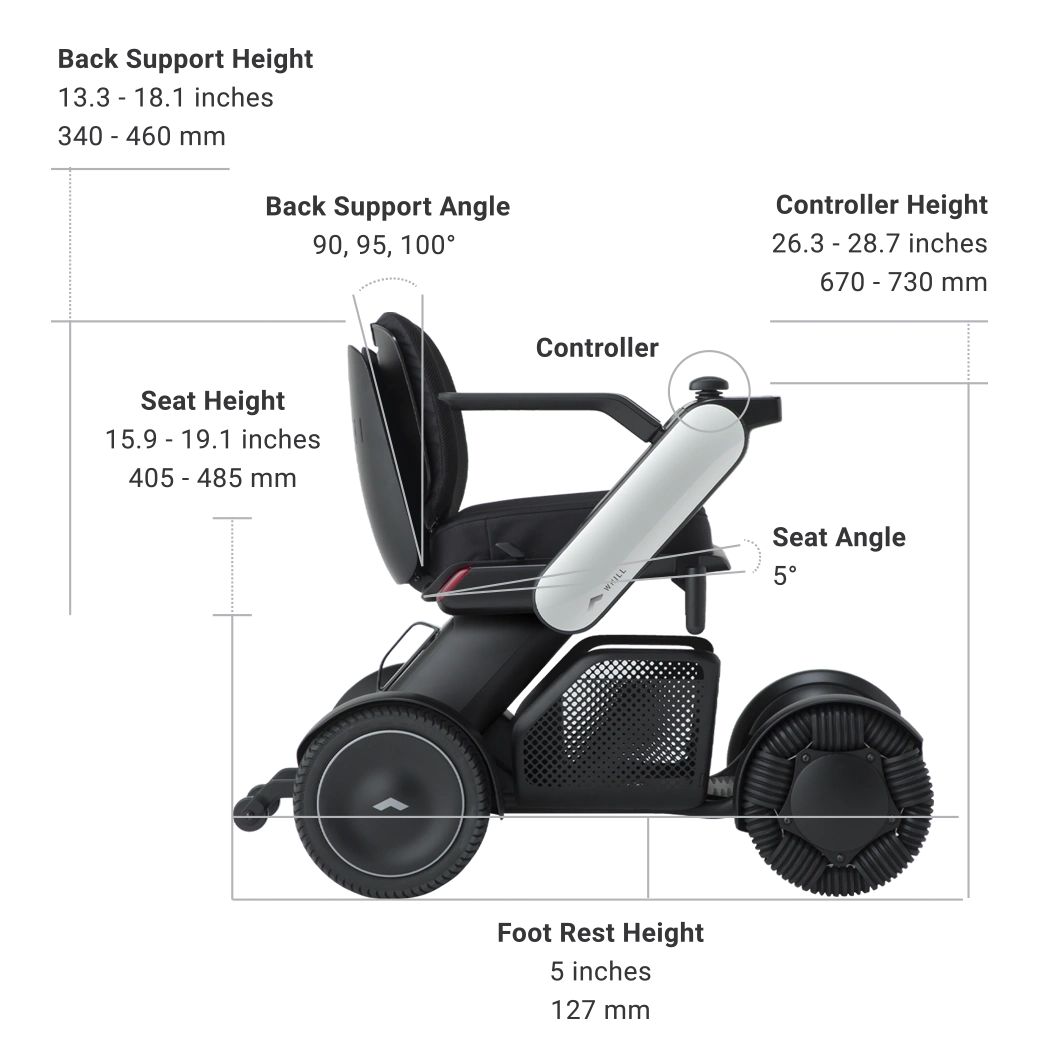
| Seat Height *6 | 15.9 to 19.1 inches (405 to 485 mm) |
| Controller Position *7 | Right, Left |
| Controller Height *8 | 26.4 to 28.7 inches (670 to 730 mm) |
| Back Support Angle | 90, 95, 100° |
| Back Support Height *9 | 13.4 to 18.1 inches (340 to 460 mm) |
*6 Seat-to-Floor height.
*7 Seat-to-Floor height.
*8 Floor to top of joystick height.
*9 Seat to top of back support cushion height.
Shipping Information
Free shipping to the contiguous US on orders over $50. Additional shipping fees may apply for Alaska and Hawaii.
Warranty
5 years: Base & Seat Frame
2 Years: Seat & Back Cushion
1 Year: Mechanical, Electrical, Components, Batteries & Tires
Awards





Accessories
By entering your email, you consent to receive promotional emails from WHILL. View our Privacy Policy for more details.
Watch Product Video
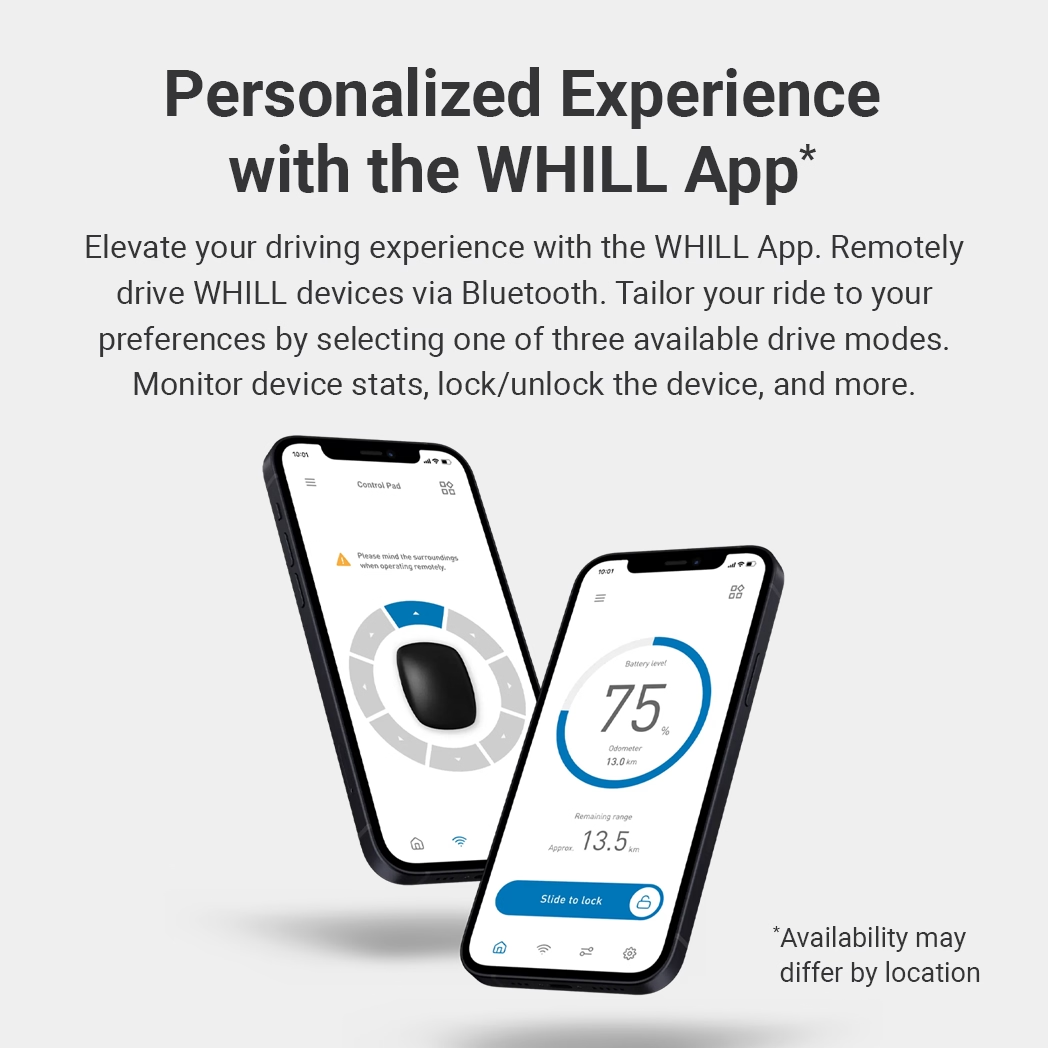
Learn More
Discover what separates the Model C2 from every other portable power chair on the market today. Explore its portability, maneuverability, and customizable comfort options that make it perfect for your active lifestyle.